In the fast-paced world of pharmaceutical and nutraceutical manufacturing, innovation isn’t just a buzzword—it’s a necessity. As consumer demands shift toward cleaner labels, vegan-friendly products, and more bioavailable formulations, liquid-filled capsules have emerged as a standout solution. Unlike traditional solid-dose forms (e.g., tablets, powder-filled capsules), liquid-filled capsules offer faster absorption, precise dosing for hydrophobic active ingredients, and improved patient compliance—especially for those who struggle to swallow large pills. But here’s the catch: the success of liquid-filled capsules hinges on one critical component often overlooked: capsule banding.
For manufacturers prioritizing plant-based, vegan, and allergen-free formulations, traditional banding solutions (such as gelatin-based adhesives) fall short. They carry risks of cross-contamination with animal-derived ingredients, fail to meet strict vegan or Halal certifications, and often lack the stability needed to seal oily or aqueous liquids over time. That’s where our breakthrough innovation comes in: 100% plant-based HPMC banding for liquid-filled capsules.
In this blog, we’ll dive deep into why liquid-filled capsules are dominating the market, the challenges of traditional banding, how our HPMC-based solution solves these pain points, and how it can help your business stay ahead of the curve. We’ll also share real-world case studies, technical specifications, and actionable insights to help you make informed decisions about your capsule manufacturing process.
1. The Rise of Liquid-Filled Capsules: Why Now Is the Time to Invest
1.3 Growth in Personalized and Functional Nutrition
- Leakage: Loss of active ingredients, contamination of other capsules, and reduced shelf life.
- Product recalls: Even minor leakage can trigger regulatory recalls, damaging brand reputation and costing millions.
- Inconsistent dosing: If liquids leak, consumers may receive less than the intended dose, compromising efficacy.
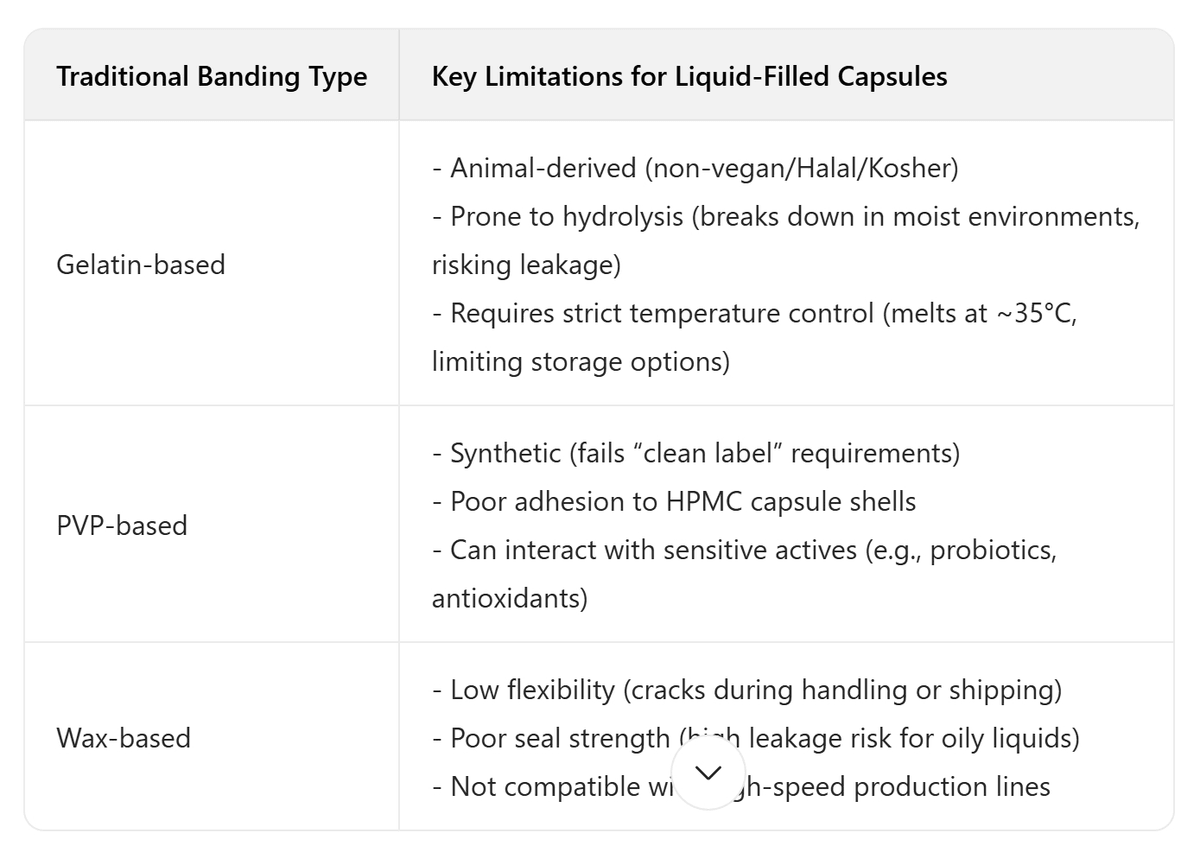
The biggest gap? None of these solutions are designed to work seamlessly with HPMC capsule shells— the most popular plant-based alternative to gelatin. HPMC capsules have a smooth, non-porous surface, which makes it harder for traditional adhesives to bond. This mismatch is why even manufacturers using HPMC shells often struggle with banding failures.
3.1 100% Plant-Derived and Clean Label-Certified
- Vegan, Halal, and Kosher certified: We hold certifications from leading bodies (e.g., The Vegan Society, Halal Certification Services, Kosher Supervision of America) to meet global market requirements.
- Clean label compliance: Free from artificial colors, flavors, or preservatives. Our formula is listed on the FDA’s Generally Recognized as Safe (GRAS) database and complies with the EU’s Regulation (EC) No 1333/2008 on food additives.
- Allergen-free: No gluten, soy, dairy, or nuts— critical for consumers with food sensitivities.
3.2 Superior Adhesion to HPMC Capsule Shells
- Adhesion strength of >5 N: Measured via tensile testing, this is 2–3x stronger than gelatin or PVP-based banding.
- Zero peeling in 6-month stability tests: Even when stored at 40°C/75% relative humidity (accelerated aging conditions).
- Compatibility with high-speed lines: Our banding dries in <30 seconds, allowing for production speeds of up to 200,000 capsules per hour— no slowdowns.
3.3 Unmatched Stability for All Liquid Formulations
- Hydrophobic modification: A proprietary additive reduces water absorption, preventing the band from swelling or breaking down in aqueous formulations.
- Oil resistance: The formula repels oils and fats, eliminating “bleeding” (where oils seep through the band and weaken the seal).
- Broad temperature tolerance: Stable from -20°C (freezer storage for sensitive actives) to 45°C (hot climates), with no cracking or leaking.
- Omega-3 fish oil (oily)
- Vitamin D3 in MCT oil (oily)
- Echinacea extract (aqueous)
- Probiotic suspension (semi-solid)
3.4 Regulatory Compliance for Global Markets
- FDA DMF 备案 (Drug Master File): For pharmaceutical customers, our DMF allows for faster regulatory approval of your finished products.
- EMA CEP 认证 (Certificate of Suitability): Complies with the European Pharmacopoeia (Ph. Eur.) monograph for HPMC, ensuring acceptance in EU markets.
- China NMPA compliance: Meets the requirements of the National Medical Products Administration, making it easy to enter the fast-growing Chinese market.
- Full traceability: We provide batch records, certificate of analysis (CoA), and raw material sourcing documents for every order— critical for audit readiness.
3.5 Cost-Effective and Easy to Integrate
- Low dosage rate: Our formula is highly concentrated, so you use 15–20% less adhesive per capsule than gelatin-based alternatives.
- Reduced waste: With 99.2% seal success rate (vs. 85–90% for traditional banding), you’ll scrap fewer batches.
- No extra equipment: No need to invest in new mixers, heaters, or applicators— our banding works with your current setup.
- Free technical support: Our team provides on-site training and process optimization to ensure a smooth transition.
4. Real-World Success: Case Study of a Nutraceutical Brand Transforming Their Liquid-Filled Capsules
- They used HPMC shells but relied on gelatin banding, which prevented them from obtaining vegan certification.
- Leakage rates were 10–12%, leading to frequent customer complaints and a 5% batch rejection rate.
- Their gelatin banding required strict temperature control (20–22°C), increasing energy costs and limiting production flexibility.
- Vegan certification achieved: Within 3 months, their omega-3 capsules were certified vegan by The Vegan Society, opening up a new market segment (vegan consumers account for 15% of their target demographic).
- Leakage rate dropped to 0.8%: Batch rejection rate fell from 5% to 0.3%, saving $120,000 annually in waste.
- Production efficiency improved: They eliminated temperature controls, increasing line speed by 10% and reducing energy costs by 8%.
- Sales growth: In the first 6 months post-launch, their omega-3 line saw a 35% sales increase— driven by the vegan certification and improved product quality.
5.1 Check for Liquid Compatibility
5.2 Verify Certifications
5.3 Evaluate Adhesion to Your Capsule Shell
5.4 Assess Production Compatibility
5.5 Look for Technical Support
6. The Future of Liquid-Filled Capsules: Why HPMC Banding Is Here to Stay
6.1 Clean Label Will Become Non-Negotiable
6.2 Personalized Medicine Will Drive Demand for Custom Formulations
6.3 Emerging Markets Will Fuel Growth
7. FAQ: Your Questions About Plant-Based HPMC Banding Answered
Q1: Is your HPMC banding compatible with gelatin capsule shells?
Q2: Can your banding be used with delayed-release (enteric-coated) capsules?
Q3: What is the shelf life of your HPMC banding?
Q4: Do you offer custom formulations?
Q5: How long does it take to receive samples?
8. Take the Next Step: Partner With Us for Plant-Based HPMC Banding Excellence
- Email: Kevin@eekpharma.com
- Website: Liquid Capsules and HPMC Banding

